CatSper is the principal Calcium channel in sperm cells, which plays a crucial role in allowing it to be able to fertilize an egg.
Intraflagellar alkalinization activates this channel, which in humans is done through the proton voltage-gated ion channel, Hv1. Miller et al., 2018 introduced a model which explains the cause of the asymmetrical bending observed in hyperactivated sperm. Hv1 is asymmetrically distributed, leading to a higher local pH around the Hv1 channels within the flagellum. This results in greater activation of the nearest CatSper channel, explaining the movement pattern.
In Lishko et al., 2011, it was shown that in human sperm, Progesterone activates CatSper, the principal Ca2+ channel. Since sperm are transcriptionally and translationally silent, this activation occurs through non-genomic steroid signally, instead of by binding to a nuclear progesterone receptor. In patients with a specific loss of CatSper function, a Calcium influx in response to progesterone is not observed. In mouse sperm, there is no Calcium influx through CatSper in response to progesterone.
The mechanism was shown in Miller et al., 2016 to occur through the binding of Progesterone to ABHD2, a serine hydrolase that is highly expressed on the flagellum of sperm. Upon binding to Progesterone, ABHD2 cleaves a 2-arachidonoylglycerol, an inhibitor of CatSper, into glycerol and arachidonic acid.
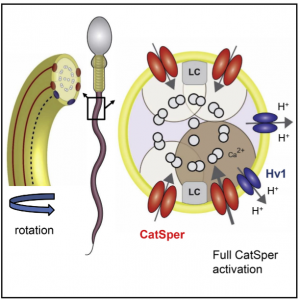
Figure from Miller et al., 2018
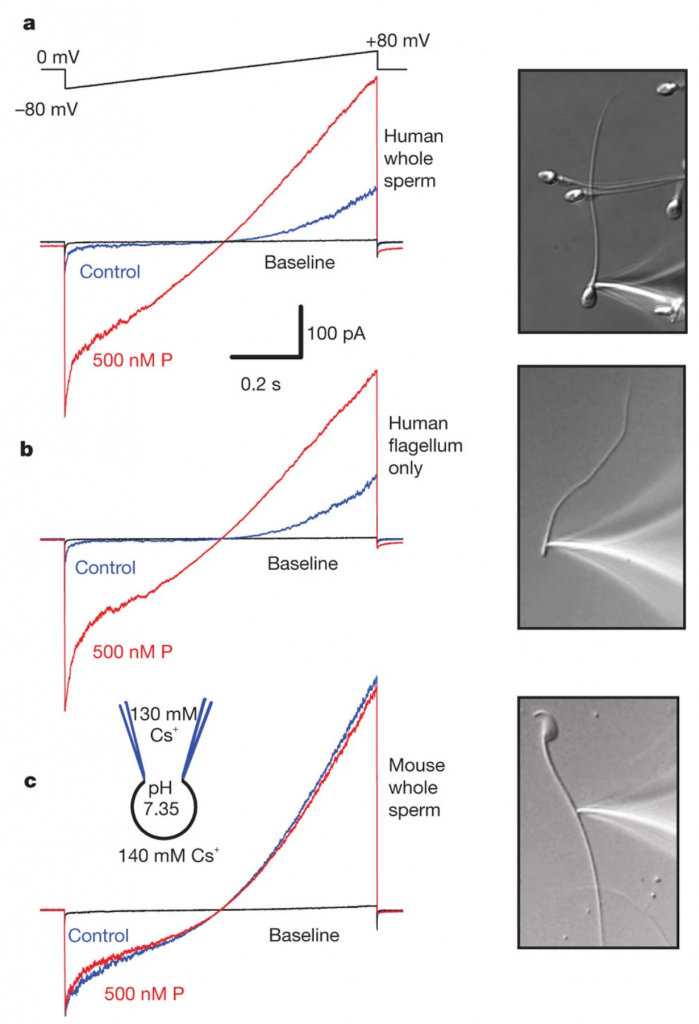
Figure from Lishko et al., 2011

